
Figure 1.
Examples of products using NiMH cells
Fortunately, with the development of new Nickel-Metal Hydride (NiMH) battery options, improvements in electronics have now been matched by significant improvements in the batteries that power them. Nickel-Metal Hydride battery cells provide more power (in equivalently sized packages) than Nickel Cadmium (NiCd) cells while also eliminating some of the environmental concerns over the use of heavy metals in the cells.
This manual provides an introduction to this exciting new battery technology while presenting recommendations for use of Nickel-Metal Hydride cells that will provide optimum results in battery-powered products.
Advantages of the Nickel-Metal Hydride
Cell
The three major benefits of the Nickel-Metal Hydride cells to
designers of portable electrical and electronic products are:
Typical Applications
The Nickel-Metal
Hydride cell is currently finding widespread application in those high-end
portable electrical and electronic products were battery performance parameters,
notably run time, are a major consideration in the purchase decision. First
adoption of the Nickel-Metal Hydride cell occurred in two markets, cellular
phones and portable computers, which are growing dramatically thanks to
significant reductions in weight and volume coupled with major improvements in
performance. The second major adoption was in the power tool market where
additional operating time and high power are of major importance. Examples of
the range of products currently powered by Nickel-Metal Hydride batteries are
shown in Figure 1 below. Penetration of the Nickel-Metal Hydride cell technology
has been strongest in premium electronic products and power tool devices that
require premium performance.

Figure 1.
Examples of products using NiMH
cells
Comparison of NiMH and NiCd CellsComparison of NiMH and NiCd Cells
Nickel-Metal Hydride cells are
essentially an extension of the proven sealed Nickel Cadmium cell technology
with the substitution of a hydrogen-absorbing negative electrode for the
cadmium-based electrode. While this substitution increases the cell electrical
capacity (measured in ampere-hours) for a given weight and volume and eliminates
the cadmium which raises toxicity concerns, the remainder of the Nickel-Metal
Hydride cell is quite similar to the Nickel Cadmium product. Many application
parameters are little changed between the two cell types, and replacement of
Nickel Cadmium cells in a battery with Nickel-Metal Hydride cells usually
involves few significant design issues. Table 1 compares key design features
between the two cell chemistries.
Table 1 - Summary Comparison of Nickel-Metal Hydride Application Features.
|
Application Feature |
Comparison of Nickel-Metal Hydride to Nickel Cadmium Batteries |
| Nominal Voltage | Same (1.25V) |
| Discharge Capacity | NiMH up to 40% greater than NiCd |
| Discharge Profile | Equivalent |
| Discharge Cutoff Voltages | Equivalent |
| High Rate Discharge Capability | Effectively the same rates |
| High Temperature (>35oC) Discharge Capability | NiMH slightly better than standard NiCd cells |
| Charging Process | Generally similar; multiple-step constant current with overcharge control recommended for fast charging NiMH |
| Charge Termination Techniques | Generally similar but NiMH transitions are more subtle. Backup temperature termination recommended. |
| Operating Temperature Limits | Similar, with NiMH performing slightly better at cold temperatures. |
| Self-Discharge Rate | Similar to NiCd |
| Cycle Life | Similar to NiCd |
| Mechanical Fit | Equivalent |
| Mechanical Properties | Equivalent |
| Selection of Sizes/Shapes/Capacities | Equivalent |
| Handling Issues | Similar |
| Environmental Issues | Reduced with NiMH because of elimination of cadmium toxicity concerns. Collection of spent NiMH batteries is not mandated. |
CELL FUNDAMENTALS
The Nickel-Metal Hydride cell chemistry is a hybrid of the proven positive electrode chemistry of the sealed Nickel Cadmium cell with the energy storage features of metal alloys developed for advanced hydrogen energy storage concepts. This heritage in a positive-limited cell design results in batteries providing enhanced capacities while retaining the well-characterized electrical and physical design features of the sealed Nickel Cadmium cell design.
Electrochemistry
The
electrochemistry of the Nickel-Metal Hydride cell is generally represented by
the following charge and discharge reactions:
Charge
At the negative electrode, in the presence of the alloy and with an electrical potential applied, the water in the electrolyte is decomposed into hydrogen atoms, which are absorbed into the alloy, and hydroxyl ions as indicated below.
Alloy + H2O + e¯ à Alloy [H] + OH¯At the positive electrode, the charge reaction is based on the oxidation of nickel hydroxide just as it is in the Nickel Cadmium couple.
Ni(OH)2 + OH¯ à NiOOH + H2O + e¯Discharge:
At the negative electrode, the hydrogen is desorbed and combines with a hydroxyl ion to form water while also contributing an electron to the circuit.
Alloy [H] + OH¯ à Alloy + H2O + e¯At the positive electrode, nickel oxyhydroxide is reduced to its lower valence state, nickel hydroxide.
NiOOH + H2O + e¯ à Ni(OH)2 + OH¯
Cell Components
Nickel-Metal
Hydride cells, with the exception of the negative electrode, use the same
general types of components as the sealed Nickel Cadmium cell.
Negative Electrode
The basic
concept of the Nickel-Metal Hydride cell negative electrode emanated from
research on the storage of hydrogen for use as an alternative energy source in
the 1970s. Certain metallic alloys were observed to form hydrides that could
capture (and release) hydrogen in volumes up to nearly a thousand times their
own volume. By careful selection of the alloy constituents and proportions, the
thermodynamics could be balanced to permit the absorption and release process to
proceed at room temperatures and pressures. The general result is shown
schematically in Figure 2 where the much smaller hydrogen atom is shown absorbed
into the interstices of a bimetallic alloy crystal structure.
Two general classes of metallic alloys have been identified as possessing characteristics desirable for battery cell use. These are rare earth/nickel alloys generally based around LaNi5 (the so-called AB5 class of alloys) and alloys consisting primarily of titanium and zirconium (designated as AB2 alloys). In both cases, some fraction of the base metals is often replaced with other metallic elements. The AB5 formulation appears to offer the best set of features for commercial Nickel-Metal Hydride cell applications.
The metal hydride electrode has a theoretical capacity approximately 40 percent higher than the cadmium electrode in a Nickel Cadmium couple. As a result, Nickel-Metal Hydride cells provide energy densities that are 20-40 percent higher than the equivalent Nickel Cadmium cell.

Figure 2.
Schematic of Metal-Alloy Crystal
Structure Within Nickel-Metal Hydride Negative Electrode
Positive ElectrodePositive Electrode
The
Nickel-Metal Hydride positive electrode design draws heavily on experience with
Nickel Cadmium electrodes. Sintered-type positive electrodes are economical and
rugged while exhibiting excellent high-rate performance, long cycle life, and
good capacity.
The balance between the positive and negative electrodes is adjusted so that the cell is always positive-limited as illustrated in Figure 3. This means that the negative electrode possesses a greater capacity than the positive. The positive will reach full capacity first as the cell is charged. It then will generate oxygen gas that diffuses to the negative electrode where it is recombined. This oxygen cycle is a highly efficient way of handling moderate overcharge currents.

Figure 3
Relative
Electrode Balances for Nickel-Metal Hydride Cell
During
Discharge/Charge/Overcharge
Electrolyte
The electrolyte
used in the Nickel-Metal Hydride cell is alkaline, a dilute solution of
potassium hydroxide containing other minor constituents to enhance cell
performance.
Separator
The material which
provides electrical isolation between the electrodes while still allowing
efficient ionic diffusion between them.
Cell Construction
The
Nickel-Metal Hydride couple lends itself to the wound construction shown in
Figure 4, which is similar to that used by present-day cylindrical Nickel
Cadmium cells. The basic components consist of the positive and negative
electrodes insulated by separators. The sandwiched electrodes are wound together
and inserted into a metallic can that is sealed after injection of a small
amount of electrolyte. In variation of this design, Nickel-Metal Hydride cells
are also being produced in prismatic versions such as that illustrated in Figure
5. The prismatic cells may fit more easily into volume-critical
applications.

Figure 4.
Schematic of Cylindrical
Cell Construction
The general internal construction of the prismatic cell is similar to the cylindrical cell except the single positive and negative electrodes are now replaced by multiple electrode sets. Thus the trade-off for improved packaging in select applications is increased complexity in cell assembly with the corresponding increases in production cost.
Both cylindrical and prismatic Nickel-Metal Hydride cells are typically two-piece sealed designs with metallic cases and tops that are electrically insulated from each other. The case serves as the negative terminal for the cell while the top is the positive terminal.

Some finished cell designs may use a plastic
insulating wrapper shrunk over the case to provide electrical isolation between
cells in typical battery applications.
Nickel-Metal Hydride cells
contain a resealable safety vent built into the top, as illustrated in Figure 6.
The Nickel-Metal Hydride cell is designed so the oxygen recombination cycle
described earlier is capable of recombining gases formed during overcharge under
normal operating conditions, thus maintaining pressure equilibrium within the
cell. However, in cases of charger failure or improper cell/charger design for
the operating environment, it is possible that oxygen, or even hydrogen, will be
generated faster than it can be recombined. In such cases the safety vent will
open to reduce the pressure and prevent cell rupture. The vent reseals once the
pressure is relieved.

DISCHARGE PERFORMANCE
The discharge behavior of the Nickel-Metal Hydride cell is generally well-suited to the needs of today’s electronic and power tool products - especially those requiring a stable voltage for extended periods of operations.Definitions of CapacityDefinitions of Capacity
The principal battery parameter of interest to
a product designer is usually the run time available under a specified equipment
use profile. While establishing actual run times in the product is vital prior
to final adoption of a design, battery screening and initial design are often
performed using rated capacities. Designers should thoroughly understand the
conditions under which a cell rating is established and the impact of
differences in rating conditions on projected performance. The standard cell
rating, often abbreviated as C, is the capacity obtained from a new, but
thoroughly conditioned cell subjected to a constant-current discharge at room
temperature faster being optimally charged. Since cell capacity varies inversely
with the discharge rate, capacity ratings depend on the discharge rate used. For
Nickel-Metal Hydride cells, the rated capacity is normally determined at a
discharge rate that fully depletes the cell in five hours. The published C value
may reflect either an average or minimum value for all cells. Typically Nickel
Cadmium cells are rated based on minimum values while Nickel-Metal Hydride cells
are rated on average values. The difference between the two values may be
significant (~ 10 percent) depending on the variability in the manufacturing
process. Many charge and discharge parameters are normalized by the C rate since
cell performance within a family of varying cell sizes and capacities is often
identical when compared on the C basis.
Equivalent CircuitEquivalent Circuit
For
purposes of electrical analysis of the battery cell, the Thevenin equivalent
discharge circuit shown in Figure 7 is often used. This models the circuit as a
series combination of a voltage source (Eo), a series resistance (Rh = the
effective instantaneous resistance), and the parallel combination of a capacitor
(Cp = the effective parallel capacitance) and a resistor (Rd = the effective
delayed resistance).

Equivalent Discharge Circuit for a Nickel-Metal
Hydride CellEquivalent Discharge Circuit for a Nickel-Metal Hydride
Cell
Eo = effective cell no-load voltage
Re = (Rh = Rd) = total effective
internal resistance
Rh = effective instantaneous resistance
Rd = effective
delayed resistance
Cp = effective parallel capacitance
E = cell
termination voltage
For steady state purposes, the cell voltage at a given current is Eo - iRe, where Re, the effective internal resistance, is the sum of Rh and Rd. The transient response is shown in Figure 8 where the initial voltage drops immediately to Eo - iReh and then transfers exponentially (with a time constant = Cp *Rd) to the steady-state voltage. Obviously the process reverses when the load is reduced or removed. For many applications, the steady-state voltage is adequate for describing cell performance since the time constant for most cells is small: usually less than 3 percent of the discharge time.

Figure 8.
Example of
Transient Voltage Profile for a Nickel-Metal Hydride Cell
Voltage During DischargeVoltage During
Discharge
The discharge voltage profile, in addition to the transient effects
discussed above, is affected by environmental conditions, notably discharge
temperature and discharge rate. However, under most conditions the voltage curve
retains the flat plateau desirable for electronics and power tool
applications.
Shape of Discharge CurveShape of Discharge
Curve
A typical discharge profile for a cell discharged at the 5-hour rate
(the 0.2C rate) is shown in Figure 9. The initial drop from an open-circuit
voltage of approximately 1.4 volts to the 1.2 volt plateau occurs rapidly.

Figure 9.
Typical Discharge
Voltage Profile for a Nickel-Metal Hydride Cell
Then, as with Nickel Cadmium cells, the Nickel-Metal Hydride cell exhibits a sharp "knee" at the end of the discharge where the voltage drops quickly. As can be seen by the flatness of the plateau and the symmetry of the curve, the mid-point voltage (MPV - the voltage when 50 percent of the available capacity is discharged) provides a useful approximation to average voltage throughout the discharge.
Environmental EffectsEnvironmental Effects
The
principal environmental influences on the location and shape of the voltage
profile are the discharge temperature and discharge rate. As indicated in Figure
10, small variations from room temperature (± 10oC) will not appreciably affect
the Nickel-Metal Hydride cell voltage profile. However major excursions,
especially lower temperatures, will reduce the mid-point voltage while
maintaining the general shape of the voltage profile.

Figure 10.
Mid-Point Voltage
Variation with Temperature
Discharge Rate
Discharge Rate
The
effect of discharge rate on voltage profile is shown in Figure 11. There is no
significant effect on the shape of the discharge curves for rates under 1C; for
rates over 1C, both the beginning and ending transients consume a larger portion
of the discharge duration.

Figure 11.
Voltage Profile
Variation with Discharge Rate
Discharge Capacity BehaviorDischarge Capacity
Behavior
As with the voltage profile, the capacity available during a
discharge is dramatically affected by the cell temperature during discharge and
the rate of discharge. The capacity is also heavily influenced by the operating
history of the cell, i.e. the recent charge/discharge/storage history of the
cell. Obviously a cell can only discharge the capacity which has been returned
to it from the previous charge cycle less whatever is lost to self discharge.
Charging/charge return issues are discussed in the next section while storage
and self-discharge is addressed in a later section.
Effect of TemperatureEffect of Temperature
The
primary effects of lower cell temperatures (<0C) on dischargeable capacity,
assuming adequate charging, are slight derating of capacity from
room-temperature values.
Effect of Discharge Rate
There is no
significant effect on capacity for discharge rates below 1C. At the discharge
rates above 1C reductions in voltage delivery occur. This voltage reduction may
also result in capacity reduction depending on the Nickel-Metal Hydride cell
design chosen and the discharge termination voltage as discussed
earlier.
Discharge Application ConsiderationsDischarge
Application Considerations
In general, the discharge behavior of Nickel-Metal
Hydride cells closely follows that of similar Nickel Cadmium cells used in the
same environment. Thus much of the design expertise gathered for Nickel Cadmium
cells is directly applicable to Nickel-Metal Hydride cells. Discussed below are
some specific issues often raised by designers using Nickel-Metal Hydride cells.
As the Nickel-Metal Hydride experience base builds, additional information that
will help designers optimize the use of Nickel-Metal Hydride cells is becoming
available. For this reason, close consultation with the factory during the
design effort is encouraged.
State-of-Charge MeasurementState-of-Charge
Measurement
A major issue for users of portable electronics is the run time
left before they need to recharge their batteries. Users of portable computers,
in particular, expect some form of "fuel gauge" to help them determine when they
need to save their work. A variety of schemes for measuring state-of-charge have
been suggested. In general, experience with Nickel-Metal Hydride cells indicates
that, due to the flatness of the voltage plateau under normal discharge rates,
voltage sensing cannot be used to accurately determine state-of-charge. To date,
the only form of state-of-charge sensing found to consistently give reasonable
results is coulometry—comparing the electrical flows during charge and discharge
to indicate the capacity remaining. Many devices already have the electronics
available to perform sophisticated tracking of charge flows including estimation
of self-discharge losses. With careful initial calibration and appropriate
compensation for environmental conditions, predictions accurate within 5 to 10
percent of actual capacity have been demonstrated. Moltech Power Systems has
developed the expertise to incorporate electronic solutions that make accurate
state-of-charge measurements possible.
Memory/Voltage DepressionMemory/Voltage
Depression
The issue of "memory" or voltage depression has been a concern for
many designers of devices, using Nickel Cadmium cells. In some applications
where Nickel Cadmium cells are routinely partially discharged, a depression in
the discharge voltage profile of approximately 150 mV per cell has been reported
when the discharge extends from the routinely discharged to rarely discharged
zones. While the severity of this problem in Nickel Cadmium cells is open to
differing interpretations, the source of the effect is generally agreed to be in
the structure of the cadmium electrode. With the elimination of cadmium in the
Nickel-Metal Hydride cell, memory is no longer a major concern.
Discharge Termination Discharge Termination
To
prevent the potential for irreversible harm to the cell caused by cell reversal
in discharge, removal of the load from the cell(s) prior to total discharge is
highly recommended. The typical voltage profile for a cell carried through a
total discharge involves a dual plateau voltage profile as indicated in Figure
14. The voltage plateaus are caused by the discharge of first the positive
electrode and then the residual capacity in the negative. At the point both
electrodes are reversed, substantial hydrogen gas evolution occurs, which may
result in cell venting as well as irreversible structural damage to the
electrodes. It should be noted that the Nickel-Metal Hydride cell, because it
uses a negative electrode that absorbs hydrogen, may actually be somewhat less
susceptible to long-term damage from cell reversal than the sealed Nickel
Cadmium cell.
The key to avoiding harm to the cell is to terminate the discharge at the point where essentially all capacity has been obtained from the cell, but prior to reaching the second plateau where damage may occur. Two issues complicate the selection of the proper voltage for discharge termination: high-rate discharges and multiple-cell effects in batteries.

Figure 12.
Nickel-Metal
Hydride Cell Polarity Reversal Voltage Profile
Voltage Cutoff at High RatesVoltage Cutoff at
High Rates
Normally discharge cutoff is based on voltage drops with a value
of 0.9 volts per cell (75 percent of the 1.2 volt per cell nominal mid-point
voltage) often being used. As can be seen in Figure 11, 0.9 volts is an
excellent value for most medium to long-term discharge applications (<1C).
However, again as seen in Figure 11, with high drain-rate usage, the change in
shape in the voltage curve with the more rounded "knee" to the curve means that
an arbitrary 0.9V/cell cutoff may be premature, leaving a significant fraction
of the cell capacity untapped. For this reason, a better choice for voltage
cutoff in high-rate applications is 75 percent of the mid-point voltage at that
discharge rate. Note, however, that this choice of end-of-discharge voltage
(EODV) is dictated only by considerations of preventing damage to the cell.
There may be end-application justification for selection of a higher voltage
cutoff with the resulting sacrifice of some potential additional capacity.
Discharge Termination in
Batteries
Normal manufacturing variation produces a range of
capacities for battery cells. As these cells are combined in batteries, the
effects of cell capacity variations are amplified by the number of cells in the
battery. Use of termination voltage based on a simple multiple of 0.9V/cell
times the number of cells may result in a weaker cell being driven into reverse
significantly before the battery reaches the termination voltage. Both charging
techniques that minimize the amount of overcharge applied to the cell and
frequent repetitive discharging of the battery may exacerbate the problem. The
result may be premature battery failure due to the damage caused by reversal of
the weak cell. Experience indicates selection of the EODV by the following
formula provides acceptable margin to minimize battery failure from repeated
cell polarity reversal:
EODV= [(MPV-150mV)(n-1)]-200mV where MVP is the single-cell mid-point voltage at the given discharge rate and n is the number of cells in the battery. Selection of the proper discharge termination voltage, especially for large batteries or complicated application profiles, should be done in consultation with the cell manufacturer.
Proper charging of Nickel-Metal Hydride cells is the key to satisfaction with their performance in any product. A successful charging scheme balances the need for quick, thorough charging with the need to minimize overcharging, a key factor in prolonging life. In addition, a selected charging scheme should be economical and reliable in use.
In general, the Nickel-Metal Hydride cell appears to be more sensitive to charging conditions than the Nickel Cadmium cell. For this reason, charging strategies should be selected and charging parameters established in consultation with the cell manufacturer. One advantage today’s application designers do have in developing chargers for Nickel-Metal Hydride cells is the increasing availability of packaged charger circuits.
Charging SummaryCharging Summary When these guidelines are followed, Nickel-Metal
Hydride cells can be quickly and reliably charged while maximizing cycle
life. Cell Behavior During ChargeCell Behavior During
Charge Voltage, Pressure, Temperature
InterrelationshipsVoltage, Pressure, Temperature
Interrelationships The voltage spikes up on initial charging then
continues to rise gradually through charging until full charge is achieved. Then
as the cell reaches overcharge, the voltage peaks and then gradually trends
down. Since the charge process is exothermic, heat is being released throughout
charging giving a positive slop to the temperature curve. When the cell reaches
overcharge where the bulk of the electrical energy input to the cell is
converted to heat, the cell temperature increases dramatically. Cell pressure,
which increases somewhat during the charge process, also rises dramatically in
overcharge as greater quantities of gas are generated at the C rate than the
cell can recombine. Without a safety vent, uncontrolled charging at this rate
could result in physical damage to the cell.
The keys to
successful charging of Nickel-Metal Hydride cells are:
Unlike discharge performance where the behavior of Nickel-Metal
Hydride cells and traditional Nickel Cadmium cells is very similar, there are
significant differences in behavior on charge between the two cell types that
relate to basic electrochemical differences. Specifically Nickel Cadmium cells
are endothermic on charge while Nickel-Metal Hydride cells are exothermic. This
difference is manifested in the interrelationships among voltage, pressure, and
temperature as discussed below.
Figure 15 sketches typical behavior of a Nickel-Metal
Hydride cell being charged at the C rate. These curves both indicate why charge
control is important and illustrate some of the cell characteristics used to
determine when charge control should be applied.
Nickel-Metal
Hydride Cell Charging Characteristics
Charge Acceptance at TemperatureCharge Acceptance
at Temperature
The effect of temperature on charging efficiency (the increase
in cell capacity per unit of charge input) is one area of difference between
Nickel-Metal Hydride and Nickel Cadmium cells. Specifically charge acceptance in
the Nickel-Metal Hydride cell (as shown in Figure 16) decreases monotonically
with rising temperature beginning below 20°C and continuing through the upper
limits of normal cell operation. This contrasts with the Nickel Cadmium cell
which has a peak in charge acceptance in the vicinity of room temperature. With
either cell type, the drop in charge acceptance at higher temperatures remains a
significant concern to product designers who are mounting the cells in close
proximity to heat sources or in compartments with limited cooling or
ventilation.
Rate Effect on Charge AcceptanceRate Effect on
Charge Acceptance
Figure 17 indicates that the charge acceptance efficiency
for the Nickel-Metal Hydride cell is improved as the charging rate is
increased.
Overcharge DetectionOvercharge
Detection
Determining when overcharge has occurred is critical to charging
schemes that minimize the amount of time spent at high charge rates in
overcharge. In turn, these efficient charging techniques are a key to maximizing
cell life, as will be discussed later. Primary charge control schemes typically
depend on sensing either the dramatic rise in cell temperature illustrated in
Figure 18 or the peak in voltage show in Figure 19.

Figure 14.
Effect of Charge
Temperature on Discharge Capacity

Figure 15.
Effect of Charge
Rate on Charge Acceptance
Charge control based on temperature sensing is the most reliable approach to determining appropriate amounts of charge for the Nickel-Metal Hydride cell. Temperature-based techniques are thus recommended over voltage-sensing control techniques for the primary charge control mechanism.
Recommended Charging RatesRecommended Charging
Rates
Today’s trend to faster charge times requires higher charge rates than
the 0.1 to 0.3C rates often recommended for many Nickel Cadmium charging
systems. Both Figure 18 and 19 indicate that fast-charge rates serve to
accentuate the slope changes used to trigger both the temperature and
voltage-related charge terminations. A charge rate of 1C is recommended for
restoring a discharge cell to full capacity. For charging schemes that then rely
on a timed "topping’ charge to ensure complete charge, a rate of 0.1C appears to
balance adequate charge input with minimum adverse effects in overcharge.
Finally a maintenance (or trickle) charge rate of 0.025C (C/40) is adequate to
counter self-discharge and maintain cell capacity.
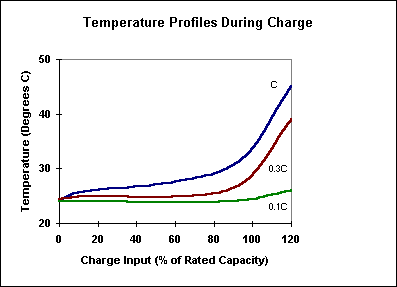
Figure 16.
Temperature
Profiles During Charge

Figure 17.
Voltage Profiles
During Charge
Effective Charging StrategiesEffective Charging
Strategies
Products using Nickel-Metal Hydride cells often make use of the
sophistication of today’s chip-level packaged charging systems to tailor the
charging profile to fast capacity recovery while minimizing overcharge stress.
Two general classes of strategies have evolved:
Two-Stage:
This approach uses a timer to switch from the initial charge rate to the maintenance charge rate. Because there is no sensing of the cell’s transition into overcharge, the charge rate must be kept low (0.1C) to minimize overcharge-related impact on cell performance and life. Charge durations are typically set at 16 to 24 hours to ensure full recharge in cases of complete discharge. Although economical, since this scheme makes no allowance for the degree of discharge or for environmental conditions, its use is rarely recommended for typical Nickel-Metal Hydride applications.Three-Stage:
Here a fast charge restores approximately 90 percent of the discharged capacity, an intermediate timed charge completes the charge and restores full capacity, then a maintenance charge provides a continuous trickle current to balance the cells and compensate for self-discharge. The fast charge (with currents in the 1C range) is typically switched to the intermediate charge using a temperature-sensing technique which triggers at the onset of overcharge. The intermediate charge normally consists of a 0.1C charge for a timed duration selected based on battery pack configuration. This intermediate-charge replaces the need to fast-charge deeply into the overcharge regime to ensure that the cell has received a full charge. Three-step charging, such as illustrated in Figure 20, requires greater charger complexity (to incorporate a second switch point and third charge rate), but reduces cell exposure to life-limiting overcharge.
Charging System RedundancyCharging System
Redundancy
Because of the sensitivity of cell life to overcharge history and
the greater subtlety of some of the overcharge transitions, charge termination
redundancy in charger design is recommended. This applies to both built-in
redundant charge control techniques and fail-safe charge termination techniques
such as thermal fusing. Both of these considerations are discussed in more
detail in the cell and battery design sections.
Temperature-Based Charge ControlTemperature-Based
Charge Control
Use of charge control based on the temperature rise
accompanying the transition of the cell to overcharge is generally recommended
because of its reliability (when compared to voltage peak sensing techniques) in
sensing overcharge. However, temperature sensing is typically more expensive to
implement than voltage sensing since it requires additional sensors. The
exothermic nature of the Nickel-Metal Hydride charge process (as illustrated in
Figure 18) results in increasing temperature throughout charging. This requires
care in selection of setpoints to avoid premature charge termination.

Figure 18.
Recommended Charge
Regime for Nickel-Metal Hydride Cells
DT/D tDT/D t
Charge switching based on the
change in slope of the temperature profile eliminates much of the influence of
the external environment and can be a very effective technique for early
detection of overcharge in a three-step charging scheme.
DTCODTCO
The simple form of temperature-based
switching is to use an absolute increment in temperature from the start of
charging, e.g. a 20°C increase in cell temperature from onset of charge. The
chosen DT has to account for both normal temperature gain during charge and the
spike at overcharge. Selection of the proper temperature increment can be
greatly influenced by the environment surrounding the cell. Thus it should be
done based on bench testing of the cell in the application and done after
consultation with the cell manufacturer.
Maximum Temperature Maximum Temperature
Charge
switching based on the absolute cell temperature (as opposed to temperature
increment) is subject to varying use patterns—Alaska or the Sahara—and is
recommended only as a fail-safe strategy to avoid destructive heating in case of
failure of the primary switching strategy.
Voltage-Based Charge ControlVoltage-Based Charge
Control
Charge control based on voltage changes is attractive because it can
be accomplished using only existing leads to the battery, eliminating the
expense and complexity of additional temperature-sensing leads to the cell.
However, the voltage peak typically occurs later in the overcharge process, the
voltage overcharge is not as distinct as that seen with temperature, and the
voltage behavior may change with cycling. For these reasons, most product
designers choose to use voltage-sensing techniques only as backups to
temperature-based control.
dV/dt
Despite the concerns voiced above,
Figure 19 does indicate a significant knee to the voltage early in overcharge
when charging at the 1C rate.. Sensing this slope change in a dV/dt (or Dv/Dt)
system can provide an effective economical approach to detecting early entry to
overcharge.
+D V+D V
Sensing the absolute voltage rise, if
carefully performed, can be a useful charge control strategy. It can be most
easily utilized if cells are usually fully discharged prior to recharge. This
approach is subject to the same caveats mentioned previously regarding
consultation and bench-level verification.
-DV-DV
Since the voltage does peak during
overcharge, switching on the voltage decrease is feasible. This eliminates the
concerns faced in both voltage and temperature increment methods about
determining the increment that ensures charge return without excessive
overcharge.
MagnitudeMagnitude
Charge control through the
absolute value of the voltage is relatively imprecise and unsuited for primary
charge-control techniques. It can be used as a redundant control technique in,
for example, a dV/dt scheme.
Time-Based Charge ControlTime-Based Charge
Control
Timer-controlled charging systems are the simplest and most
economical of all charging strategies. However, to avoid adverse effects on cell
life and performance, charging rates must be limited to 0.1C, which constrains
time-based charging to those products where overnight return of charge is
acceptable. In typical application scenarios where the degree of discharge
varies widely, a charging system using time as the primary control variable will
either undercharge or overcharge the battery. However, time-based redundant
charge termination and/or time-based control of intermediate charging (topping
charge) in a three-step system are often key elements of an integrated
charge-control strategy.
Environmental Influences on Charging
StrategyEnvironmental Influences on Charging Strategy
The discussions
above are most pertinent for devices operating in the room-ambient range.
Designers of products predominantly operating at either temperature extreme
should consult closely with their cell suppliers in designing their charging
system.
High TemperatureHigh Temperature
Although
high-temperature performance (in the 40 to 55°C range) is equivalent or even
slightly better than the standard Nickel Cadmium product, charging of
Nickel-Metal Hydride cells in high-temperature environments requires careful
attention for two reasons: (1) the selection of setpoints, for both temperature
and voltage-sensing systems, can be affected if the cells are already at
elevated temperatures prior to starting charge; and (2) charge duration may have
to be extended due to the charge acceptance inefficiencies illustrated in Figure
16.
Low TemperatureLow Temperature
Even though low
temperature charge acceptance is better for the Nickel-Metal Hydride cell than
for Nickel Cadmium cells, designers must ensure that low temperatures do not
adversely affect their charge-control scheme. The charge time increases at lower
temperatures so charge durations must be carefully considered to provide
adequate low-temperature charging while avoiding excessive charge at normal
temperatures. Charge rates must also be reduced at low temperatures. Charging
below 0°C is not advisable. Consult the factory for more details on
low-temperature charging.
Available Battery Charging SystemsAvailable
Battery Charging Systems
Traditionally, application designers tailored their
charging system to their application. With the rapid evolution of chip-based
charging circuitry, designers can now use standardized designs providing a
sophisticated charging scheme while allowing the designer wide latitude in
selecting charge parameters. Such systems are available from a variety of
sources including both cell manufacturers and integrated-circuit design houses,
in forms ranging from basic chip to complete charger packages.
STORAGE
Essentially all rechargeable battery cells gradually discharge over time whether they are used or not. This capacity loss is typically due to slow parasitic reactions occurring within the cell. As such, the loss rate (self-discharge rate) is a function of the cell chemistry and the temperature environment experienced by the cell. Due to the temperature sensitivity of the self-discharge reactions, relatively small differences in storage tempeature may result in large differences in self-discharging rate. Extended storage with a load connected not only speeds the discharge process, but may also cause chemical changes after the cell is discharged, which may be difficult or impossible to reverse.
Cell and battery storage issues of concern to most application designers relate either to the speed with which the cells lose their capacity after being charged or the ability of the cells to charge and discharge "normally" after storage for some period of time. In both situations, general guidelines developed for Nickel Cadmium cells will work acceptably for Nickel-Metal Hydride cells.
Retained CapacityRetained Capacity
Figure 21
illustrates the amount of capacity available from Nickel-Metal Hydride cells
after standing for a given number of days in four different thermal
environments. The common rule of thumb for Nickel Cadmium cells that a 10°C
increase in storage temperature halves the time required for a cell to
self-discharge to a given level remains approximately correct for Nickel-Metal
Hydride cells.

Figure 19.
Self-Discharge
Characteristics for Nickel-Metal Hydride Cells
Recommended Storage Conditions
Capacity Recovery After Storage
Capacity
Recovery After Storage
In normal practice, stored cells will provide full
capacity on the first discharge after removal from storage and charging with
standard methods. Cells stored for an extended period or at elevated
temperatures may require more than one cycle to attain pre-storage capacities.
Consultation with the manufacturer is recommended if prolonged storage and rapid
restoration of capacity is planned.
Loaded StorageLoaded Storage
Cells and
batteries intended for storage for extended periods of time (pass the point
where they are fully discharged) should be removed from their load. In
particular, many portable electronic devices place a very low-level drain
requirement on their batteries even when in the "off" position. These
micro-current loads may be sustaining volatile memory, powering sense circuits
or even maintaining switch positions. Such loads should be eliminated when
storing devices for protracted periods.
When both Nickel-Metal Hydride cells and Nickel Cadmium cells are stored under load, small quantities of electrolyte can ultimately begin to seep around the seals or through the vent. This creep leakage may result in the formation of crystals of potassium carbonate, which detract cosmetically from the appearance of the cell. In extreme cases, creep leakage can result in corrosion of cells, batteries, or the adjoining componetry. Although such occurrences are rare, positive methods of electrically isolating the cell, such as an insulating tape over the positive terminal or removal from the product, are suggested for applications requiring extended storage of cells.
A key determinant of the economic and practical feasibility of using Nickel-Metal Hydride cells and batteries in portable electronic applications is the cell’s cycle life: the ability of the Nickel-Metal Hydride cell to deliver acceptable capacity on a repetitive basis. Nickel-Metal Hydride cell cycle life has received intensive development attention with the result that operational life expectations are now competitive with those for Nickel Cadmium cells.
Limiting Mechanisms Limiting Mechanisms
The life of any battery cell is determined by a
combination of abrupt failure events and gradual cell deterioration. With the
Nickel-Metal Hydride cell, abrupt failures, typically mechanical events
resulting in the cell either shorting or going open-circuit, are relatively rare
and randomly distributed.
Cell deterioration can take two forms:
Both phenomena result in a loss of usable capacity, but pose differing design issues. Mid-point voltage depression requires that the application design be able to adapt to variations in supply voltage from cycle to cycle. Capacity reduction simply requires that initial cell selection be sized to provide adequate capacity at end-of-life for the desired number of cells. The actual mechanism that will determine cell life may vary depending on application parameters and the cell characteristics. Development work has reduced oxidation in the negative electrode reducing the depression in MPV as the cell ages.
Factors Affecting Life Factors Affecting
Life
The way the Nickel-Metal Hydride cell is designed into an application
can have dramatic effects on the life of the cell. This is especially true of
the design of the charging circuitry for the application to ensure adequate
return of charge while minimizing overcharge. In fact, effective control of
overcharge exposure, time and charge rate is the way of enhancing cell
life.
Charge Regime Charge Regime
In general,
tailoring the charge regime to the application use scenario is even more
important with Nickel-Metal Hydride cells than with Nickel Cadmium cells because
of the increased subtlety of the voltage and temperature indications of full
charge and the greater sensitivity of cell life to overcharge history.
Degree of Overcharge Degree of
Overcharge
Establishing the appropriate degree of overcharge for a
battery-powered application is dependent on the usage scenario. Some overcharge
of the battery is vital to ensure that all cells are fully charged and balanced,
but maintenance of full charge currents for extended periods once the cell has
reached full charge can reduce life. The three-step charge process works to
minimize some of the overcharge stress. Details of the charging process and the
application context should be carefully reviewed with the cell manufacturer to
ensure maximum cell life for the specific application.
Exposure to High Temperatures Exposure to High
Temperatures
In general, higher temperatures accelerate chemical reactions
including those which contribute to the aging process within the battery cell.
High temperatures are a particular concern in the charging process as charge
acceptance is reduced. Sensing the transition from charge to overcharge is also
more difficult at higher temperatures. Although early data indicate that
Nickel-Metal Hydride cells may tolerate high-temperature charging better than
standard Nickel Cadmium cells, close consultation with the cell manufacturer is
encouraged to select a charging strategy that meets operational requirements
while maximizing cell life.
Cell Reversal Cell Reversal
Discharge of
Nickel-Metal Hydride batteries to the degree that some or all of the cells go
into reverse can shorten cell life, especially if this overdischarge is repeated
routinely.
Prolonged Storage under Load Prolonged Storage
under Load
Maintaining a load on a cell (or battery) past the point of full
discharge may eventually cause irreversible changes in the cell chemistry and
promote life-limiting phenomena such as creep leakage.
DESIGNING FOR NICKEL-METAL HYDRIDE CELLS
Incorporation of Nickel-Metal Hydride cells into applications is generally straightforward, particularly for designers accustomed to designing with Nickel Cadmium cells. Primary differences between the two cell chemistries are:
Materials of ConstructionMaterials of
Construction
The materials of construction for the Nickel-Metal Hydride cell
external surfaces are, like the Nickel Cadmium cell, largely comprised of
nickel-plated steel, and therefore, are resistant to attack by most
environmental agents.
OrientationOrientation
Nickel-Metal Hydride
cells will operate satisfactorily in any orientation.
Environmental SuitabilityEnvironmental
Suitability
The Nickel-Metal Hydride cell is designed to operate effectively
in all environments normally experienced by portable electronic equipment.
Application designers intending to use Nickel-Metal Hydride cells in especially
adverse environments should consult closely with the cell manufacturer to ensure
design suitability.
TemperatureTemperature
Like most other battery
cells, Nickel-Metal Hydride cells are most comfortably applied in a
near-room-temperature environment (25°C); however, with careful attention to
design parameters, they can be successfully utilized when exposed to a much
wider range of temperatures.
OperatingOperating
Nickel-Metal Hydride cells
can be successfully applies in temperatures from -20°C to 50°C with appropriate
derating of capacity at both the high and low ends of the range. Design charging
systems to return capacity in high or low temperature environments without
damaging overcharge requires special attention.
StorageStorage
Cells are best stored in
temperatures from -40°C to 30°C although storage for limited periods of time at
higher temperatures is feasible.
Shock and VibrationShock and Vibration
Expect
Nickel-Metal Hydride cells to easily withstand the normal shock and vibration
loads experienced by portable electronic equipment in day-to-day handling and
shipping. Consult with the cell manufacturer regarding applications required
operation in more intense shock and vibration environments.
Ventilation and IsolationVentilation and
Isolation
The primary gas emitted from the Nickel-Metal Hydride cell when
subjected to excessive overcharge is hydrogen as opposed to oxygen for the
Nickel Cadmium cell. Although venting of gas to the outside environment should
not occur in a properly designed application, isolation of the battery
compartment from other electronics (especially mechanical switches that might
generate sparks) and provision of adequate ventilation to the compartment are
required to eliminate concerns regarding possible hydrogen ignition. Isolation
of the battery from heat-generating componetry and ventilation around the
battery will also reduce thermal stress on the battery and ease design of
appropriate charging systems.
Termination
Since the exterior of the
Nickel-Metal Hydride cell is nearly identical to that of the Nickel Cadmium
cell, all termination procedures accepted for the Nickel Cadmium cell apply
equally well to the Nickel-Metal Hydride cell. The recommendation against use of
mechanical (pressure) contacts in favor of welded terminations, especially to
Nickel-Metal Hydride cells. The prohibition against soldering directly to the
cell to prevent heat damage to plastic seal components also applies.
Nickel-Metal Hydride cells are versatile performers easily adapting to most application demands. Existing design libraries for Nickel Cadmium cells can usually be easily modified to incorporate Nickel-Metal Hydride cells instead. Economical off-the-shelf designs can be tailored to the specific voltage, space, and termination requirements of an application. Figure 23 illustrates a typical battery installation within a representative application, while Figure 24 diagrams many of the components recommended for a nickel-metal battery.

Figure 20.
Installation
Within Typical Application (Notebook Computer)

Figure 21.
Elements of
Battery Assembly
Packaging ConsiderationsPackaging
Considerations
Nickel-Metal Hydride batteries are generally packaged in two
forms:
- Hard plastic cases are recommended for applications requiring the end-user to handle the battery. These cases offer greater protection against handling damage and shock and vibrations stresses. But depending on the design, thermal management may be more difficult within the hard case. Injection molding of hard cases requires a substantial investment for mold construction and is thus best suited for high volumes.
- Lighter shrink-wrapped plastic packaging may be used when routine battery removal is not expected. These packs, as illustrated in Figure 24, usually consist of the cell assembly with insulators covering the exposed terminals. Plastic shrink tubing then covers the whole pack. Shrink-wrapped batteries have acceptable mechanical integrity for assembly, and when properly secured, withstand normal portable-product shock and vibration levels. Shrink packaging provides ample opportunity for hydrogen to diffuse and for internally generated heat to dissipate. Additional insulation from heat my be needed at the tangent points within the cell stacks (where they shrink material directly contacts the cell).
Either type of packaging must maintain adequate ventilation to the individual cells while providing room for cell interconnections, battery terminations, and requisite charge control sensors.
ShapeShape MaterialsMaterials Wires: All wire insulation should be Teflonâ ,
Kaptonâ , or other material with a minimum temperature rating of
200°C. Sleeving: All shrink sleeving should be able to
withstand 200°C. PVC sleeving is not generally recommended. Kraft paper or
fishpaper sleeving should be approximately 0.007 inches thick. Insulation: All cell insulation should be able to
withstand 105°C for 24 hours. Vent shields must be constructed of Nomexâ or
other insulating material capable of withstanding 210°C. Case Material: Plastic cases must meet UL 9V40.
Case materials without a rating of 210°C DTUL (Deflection Temperature Under
Load) must be provided with vent shields over the positive ends of the
cells. Interconnections and TerminationsInterconnections
and Terminations Battery terminations come in a variety of
configurations ranging from simple flying leads (wires soldered to weld lugs
which are then welded to the cells) in permanent installations to much more
elaborate contact or connector systems on removable battery packs. Removable
battery packs should be designed with a connection system that produces a
minimum of 2 pounds of force while incorporating a wiping action on insertion to
cut through oxide layers on the connection surfaces. Other ComponentsOther Components PTC Resistor: Positive temperature coefficient
resistors such as Raychem’s PolySwitchâ circuit protector provide a latching,
but resettable device for protection against short-circuit
conditions. Thermostat: Thermostats or other resettable thermal
control devices are typically used for backup to the primary charge control
system to guard against extended overcharge and the resulting elevated
temperatures. Thermal Fuse: Thermal fuses that open at a suitably
elevated temperature (nominally 90°C) are often used as a third tier of
thermal protection (after the normal charge control system and thermostat).
They are a fail-safe measure since the battery charging system will become
inoperative. Thermistor: Thermistors are normally used for the
temperature-sensing necessary for recommended charge control
schemes. Standard ConfigurationsStandard
Configurations As a minimum, Moltech Power Systems, Inc. recommends
that the following be included in any standard battery design:
LocationLocation
Battery shapes can be adjusted to
fit application constraints. Among the most popular battery shapes are the
following:
Materials used in the
assembly of Nickel-Metal Hydride batteries must withstand the high temperature
environment that accompanies venting of the cell. Because of the exothermic
nature of the charging process, should cells vent in overcharge, the vented
gases will be largely high-temperature hydrogen (>200°C). Although these
gases will quickly disperse and cool, all materials used in cell construction
must be capable of withstanding elevated temperatures while remaining inert in a
hydrogen environment. Recommended materials for use in Nickel-Metal Hydride
battery construction include those below. Consult with the cell manufacturer
regarding specific material specification details.
Cell interconnections typically consist of nickel (Ni200)
strip spot-welded from one cell terminal to the adjacent cell’s case. Nickel bus
strips offer good conductivity, ease of welding, and resistance to corrosion.
Minimum recommended nickel strip size is 0.187 inches wide by 0.005 inches
thick. Wire interconnections are rarely used because of the difficulty in
attachment since soldering directly to cells is forbidden.
Nickel-Metal
Hydride batteries typically require more components than Nickel Cadmium
batteries because of the emphasis on careful, redundant charge control including
adequate fail-safe charge termination in case of excessive temperatures. These
components include the following:
A wide variety of standard battery configurations have been
developed by cell manufacturers encompassing permutations of cell size/capacity,
voltage, terminations, and charge control and termination sensors.
While battery location is
generally influenced by product design constraints such as available space,
influence on center of gravity, and ease of access, battery locations should
also provide adequate ventilation, isolation from ignition sources and
separation from major heat generators.
CARE AND HANDLING
Nickel-Metal Hydride cells should be handled in much the same manner as Nickel Cadmium cells. Major points are summarized below. Contact the cell manufacturer for additional information pertinent to specific applications.
General Safety PrecautionsGeneral Safety
Precautions Shipping and HandlingShipping and
Handling DisposalDisposal Incoming Inspection Normal incoming inspection techniques consist of
physical examination of the cells for any dents, bulges, or leakage and
selection of a representative sample for capacity testing. In general 100
percent capacity testing is discouraged because of the cost/schedule impact.
Specialized incoming test procedures are normally developed for each application
by consultation between the product designer and the cell
manufacturer. This reference manual contains general
information on all Moltech Power Systems, Inc. batteries within the Nickel-Metal
Hydride chemical system in production at the time of publication of the manual.
Since the characteristics of individual batteries are sometimes modified,
persons and businesses that are considering the use of a particular battery
should contact the nearest Moltech Power Systems sales office for current
information. None of the information in this manual constitutes a representation
or warranty by Moltech Power Systems concerning the specific performance or
characteristics of any of the batteries or devices. © 2000 Moltech Power Systems, Inc
Nickel-Metal Hydride cells are generally well-behaved; however,
like any rechargeable cell, they should be treated with care. Issues in dealing
with Nickel-Metal Hydride cells include the following:
Shipping and handling of Nickel-Metal Hydride cells is
straightforward. The following suggestions ensure maximum performance,
reliability, and safety in working with the cells:
Although disposal procedures
for Nickel-Metal Hydride cells are still evolving, as a minimum, observe the
following precautions: